CAR-T Cell Therapy: A Breakthrough in the Treatment of Hemato-Oncological Diseases
Based on an article by Prof. Arnon Nagler, director of the Hematological System, Bone Marrow Transplantation, and Cord Blood Bank at the Sheba Medical Center in Israel. (2021)Cancer is a disease that affects millions of people worldwide, with blood cancers being among the most difficult to treat. Despite significant advancements in medical technology, treating hematological malignancies, such as leukemia and lymphoma, remains a significant challenge. Traditional treatments like chemotherapy and radiation therapy have been the go-to options for many years, but they come with many limitations, including side effects and the potential for drug resistance. However, recent developments in cellular immunotherapy, specifically chimeric antigen receptor T-cell therapy, also known as CAR-T cell therapy, have opened up new possibilities for treating these challenging cancers.
CAR-T cell therapy is a form of gene therapy that involves engineering a patient's own T-cells to attack and destroy cancer cells. This innovative technology has been hailed as the most promising and revolutionary innovation in the field of hematology in recent times. The therapy's development has been a significant milestone in the treatment of hematological malignancies, especially for those who have exhausted all other available options. This article will discuss the mechanism of action of CAR-T cell therapy, its advantages and limitations, side effects, and the progress that has been made in the field.
CAR-T Cell Therapy: A Brief History
The CAR-T cell technology was first developed about ten years ago at the Weizmann Institute. The first protein against which CAR-T cells were synthesized was 19CD, found on the surface of healthy and malignant B cells. The first two patients treated were chronic lymphocytic leukemia (CLL) patients, who were treated with extraordinary success. Over 100 patients have been treated with CAR-T cell therapy, and 90% of these patients experienced a complete response. In later studies, patients with different types of lymphoma that had exhausted other lines of treatment were treated with CAR-T cell therapy, and 70% of these patients responded to the treatment.
Standard Treatments for Hemato-Oncological Diseases
Chemotherapy is the first line of treatment for malignant hematological diseases. Two immunotherapies, Blinatumomab Blincyto and Inotuzumab Besponza, have been approved for patients who do not respond to chemotherapy. Patients who do not respond to treatment are advised to undergo allogeneic bone marrow transplantation, which can be supplemented with biological therapy or immunotherapy.
Mechanism of Action of CAR-T Cells
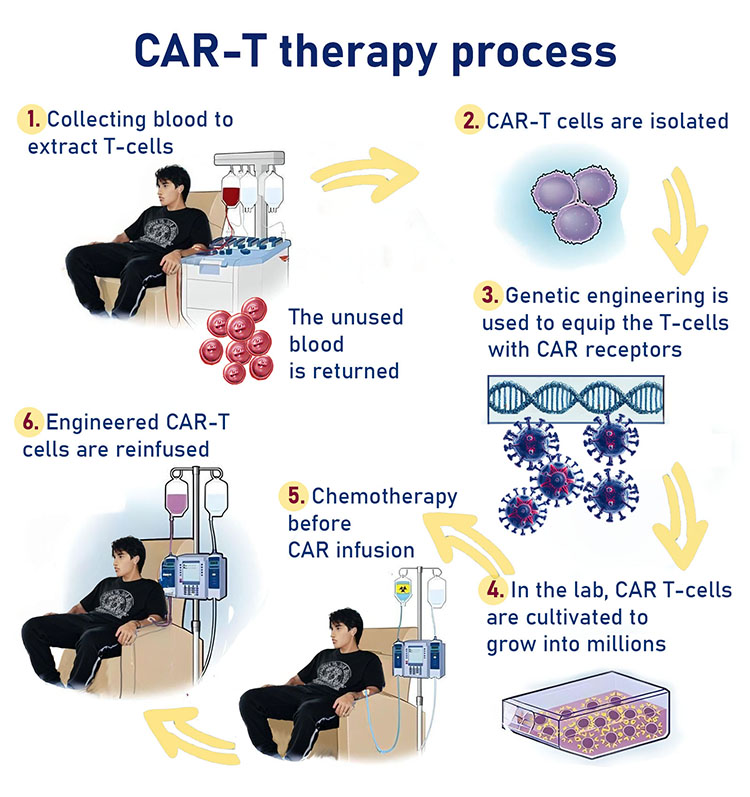
CAR-T cell technology is a cellular immunotherapy that affects only the cells that express a specific antigen on the surface of the cell. In this treatment, an autologous T cell transplant is performed on the patient, and the T cells are engineered to express a chimeric antigen receptor (CAR) that targets specific proteins. The patients undergo chemotherapy against the natural T cells that remain in the body and are then given an infusion of the transgenic cells. The high specificity of CAR-T cells enables the proliferation of T cells, resulting in the withdrawal of malignant diseases associated with B cells, without damage to cells that do not express the antigen.
Side Effects of CAR-T Cell Therapy
CAR-T cell therapy is associated with a number of side effects, including a decrease in the number of lymphocytes and immunoglobulins, a neurological disorder that affects 15% of the patients, and cytokine release syndrome (CRS). CRS is a systemic inflammatory reaction that occurs due to cellular activation and proliferation, which can range from mild to severe, causing an increase in cytokine levels, which in severe cases can result in hypotension and pulmonary edema.
Conclusion
CAR-T cell therapy has shown promising results in treating hemato-oncological diseases, and it is expected that the treatment will be available to more patients soon. However, the treatment is associated with a number of side effects that must be considered, and further research is necessary to improve the efficacy and safety of the therapy.