CAR T in Sheba: Clinical trials results for relapsed or refractory acute lymphoblastic leukemia (ALL) and non-Hodgkin's lymphoma (NHL)
Source: National Library of Medicine, published March 2020.
This study focuses on the efficacy of CD19 chimeric antigen receptor T (CAR-T) cells in treating both pediatric and adult cases of refractory or relapsed (r/r) acute lymphoblastic leukemia (ALL) and non-Hodgkin's lymphoma (NHL). Beginning in 2016, a trial featuring in-house created CD19 CAR-T cells with CD28 co-stimulatory domains was launched. This trial marked the first comparative analysis of production characteristics and phenotypic variances in CAR-T cells between ALL and NHL patients.
Study Design
The process involved using patients' non-cryopreserved peripheral blood mononuclear cells to produce CAR-T cells in a span of 9 to 10 days. The study enrolled 93 patients with r/r ALL and NHL. Simultaneous production of CAR-T cells for both ALL and NHL patients enabled direct comparisons.
Findings
Despite all participants having undergone extensive prior treatments, three withdrew due to clinical worsening or production issues. It was observed that ALL patients' cells (37 in number) showed greater expansion and higher CAR-T cell counts compared to those from NHL patients (53 in number). Younger patients exhibited better cell proliferation. ALL patient infusions had more naive CAR-T cells and higher CXCR3 expression, while markers like PD-1, LAG-3, TIM-3, and CD28 were uniformly expressed. The study achieved its target dose in all ALL patients and 94% of NHL patients. The response rate was 84% in ALL and 62% in NHL. The study also compared CAR-T cell infusions to tumor infiltrating lymphocytes (TIL), commonly used in solid tumors, finding more naive and central memory T cells and less CCR5 in CAR-T cells.
Conclusion
The rapid and efficient in-house CAR-T cell production is notable, with a high clinical response rate. Nearly all patients (99%) received CAR-T cells within 9 to 10 days. ALL-derived cells showed superior proliferation and higher naive T cell frequencies than NHL-derived ones. These findings suggest potential avenues for adapting CAR-T cell therapy for solid tumors based on the differences observed with TIL infusion products.
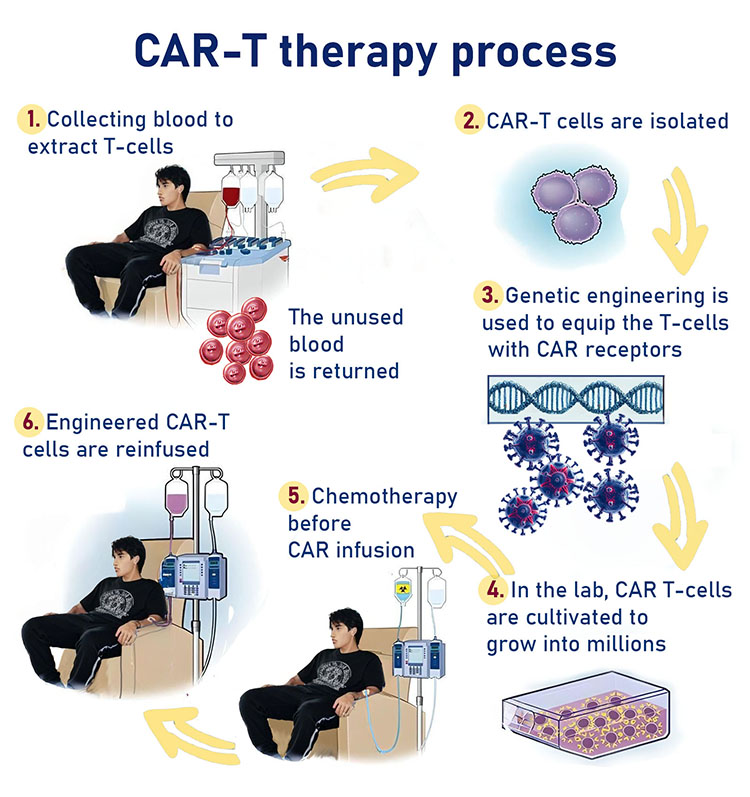
CAR-T information:



