Encouraging Efficacy of Point-of-Care Anti-BCMA CAR T-Cell Therapy in High-Risk Relapse/Refractory Multiple Myeloma
Date of publication: April 2023
Led by Hila Magen, recent clinical trials have showcased promising response rates using point-of-care anti-BCMA CAR T-cell therapy in treating relapse/refractory multiple myeloma (R/R MM). This innovative approach is especially noteworthy for its application in high-risk patient cohorts who have exhausted standard therapeutic lines.

Left to right: Dr Arnon Nagler, Dr Abraham Avigdor, Dr Hila Magen.
Technical Overview of the Therapy
This novel treatment modality involves the autologous engineering of T-cells to express anti-BCMA chimeric antigen receptors (CARs). The point-of-care (POC) manufacturing process bypasses the need for cryopreservation and logistics of cell transport, thus streamlining the CAR T-cell production and reducing the lead time to infusion.
Clinical Trial Insights
The phase 1b/2 trial (NCT05243212) detailed the process, where patients underwent leukapheresis, followed by isolation and activation of T-cells. These cells were then transduced with a retroviral vector encoding the anti-BCMA CAR, featuring 11D5-3 ScFv, CD28 costimulatory, and CD3-ζ signaling domains. Pre-infusion lymphodepletion was achieved with fludarabine and cyclophosphamide, followed by the infusion of CAR T-cells at specified dose levels.
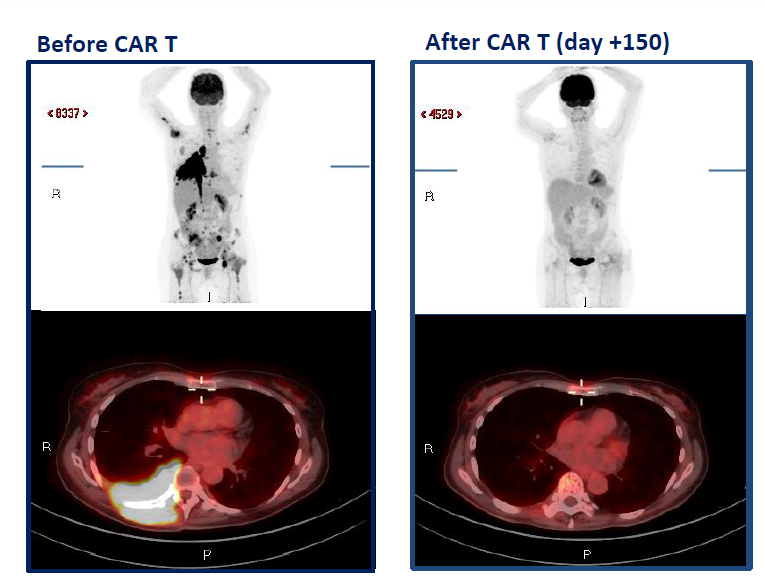
Outcomes and Safety Profile
The trial reported a favorable safety profile, with minimal cases of grade 3 cytokine release syndrome and no immune effector cell-associated neurotoxicity syndrome observed. Hematologic toxicities were within manageable ranges. The overall response rate and progression-free survival metrics align with the therapeutic efficacy noted in similar cohorts.
Clinical Implications
This therapy is a significant stride forward for R/R MM patients, particularly those ineligible for other trials. The rapid turnaround time of CAR-T cell production is a pivotal advantage, reducing the dependency on bridging therapies. However, the diminished efficacy in patients with prior exposure to BCMA-targeted therapies highlights the need for strategic sequencing in multiple myeloma management.
Conclusion
Point-of-care anti-BCMA CAR T-cell therapy has emerged as a potent option for high-risk MM patients, demonstrating high response rates and a commendable safety profile. The study underscores the potential shifts in treatment paradigms and the necessity of personalized therapy sequencing in multiple myeloma.
Sources: National Library of Medicine
BISPECIFIC ANTIBODIES - Advanced treatment for Myeloma patients >>
CAR-T information:



