What is the difference between Sheba’s In-house CAR-T therapy and other commercial CAR-T’s?
As of 2022, there are six CAR-T treatments available around the world, approved by the FDA and manufactured by pharmaceutical companies such as Novartis, Gilead, Kite, Promab, Lonza and more. They are:
- Abecma™ (idecabtagene vicleucel)
- Breyanzi™ (lisocabtagene maraleucel)
- Kymriah™ (tisagenlecleucel)
- Tecartus™ (brexucabtagene autoleucel)
- Yescarta™ (axicabtagene ciloleucel)
- Carvykti™ (ciltacabtagene autoleucel)
These CAR- T’s are designed to be integrated in the blood of the patient, which is shipped to the manufacturer from anywhere on the globe, and shipped back to the patient in a frozen condition to preserve freshness. This process can take 1-2 months. As opposed to this procedure, the Sheba in house CAR T cell therapy is manufactured locally in the Sheba advanced laboratories and delivered to patient immediately, within 10 days. This procedure is possible due to Sheba’s cooperation with Lonza, an established expert in autologous cell-therapy development and manufacturing, using their automated CocoonTM platform, which was recreated in Sheba hospital in Israel. By using Cocoon’s automated cell manufacturing process, Sheba can promote a higher standard of cell quality and lower it’s production cost. In-house manufacturing of car-t cells leads to treatments that are similarly effective to commercially manufactured therapies, in much less time.
 Lonza’s cocoon system at Sheba hospital
Lonza’s cocoon system at Sheba hospital
Comparison of CAR-T processes
| SHEBA IN HOUSE CAR-T | OTHER COMMERCIAL CAR-T’S |
|---|---|
| The entire process is done in-house | Blood & cells fly on a long journey overseas |
| Cell quality is preserved | Cell quality affected by the freezing |
| Suitable for a wide range of diseases | Approved for a few diseases |
| Takes about 10 days | Takes 1-2 months |
| Cost: $80K-$120K USD | Cost: $200K-$500K USD (Depending on the country) |
CAR-T therapy consultation in Sheba hospital

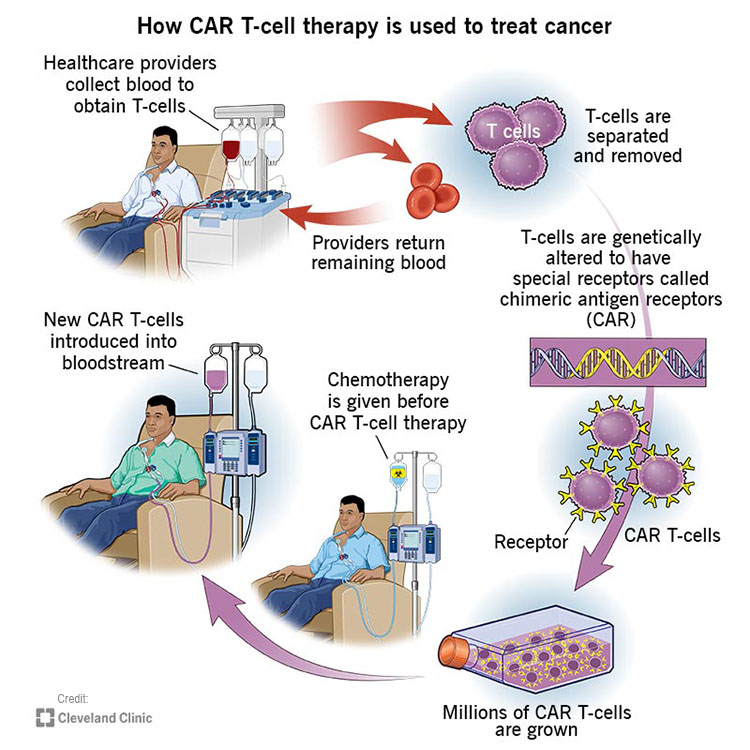
What are the steps of the CAR T therapy in Sheba?
- Patients send medical documents to check initial eligibility.
- Patient arrives to Sheba, performs test and screening to determine final eligibility. (10-14 days)
- Blood collection- Patients blood is collected, and T cells are separated from the rest of the blood. The blood is returned to the patient.
- Genetic engineering – The T cells are engineered in Sheba’s lab to produce CAR’s on their surface.
- Growing T-cells-the cells are multiplied in the Sheba laboratory. (Approximately 7 days)
- Preparing for infusion- before the CAR-T cells are returned, light chemotherapy is administered to the patient.
- The T-cells are returned to the patient.
- Hospitalization period 2-3 weeks-the patient remains in hospitalization in order to monitor possible side effects.
- PET CT scan- approximately 30 days after the CAR-T cells are returned, a Pet CT scan is performed to check the regression of the disease.
- The standard protocol of the whole process takes 10-12 weeks.
READ MORE ABOUT CAR-T THERAPY IN SHEBA HOSPITAL >>
CAR-T therapy in Sheba hospital, Israel - patient's father review
CAR-T information:
- CAR-T cell therapy in Sheba hospital
- Car-T for Multiple Myeloma at Sheba summary
- Sheba CAR-T cell therapy activity 2023
- Cost of CAR-T cell therapy in Sheba
- Understanding the costs of car-t therapy at Sheba
- The difference between Sheba’s in-house CAR-T therapy and other commercial CAR-T’s
- Why is the car-t cell therapy at Sheba superior
- CAR-T in Sheba for ALL and NHL
- CAR-T success rate in Sheba, Israel - 2024 activity overview
- About CAR-T clinical academical trial in Sheba
- CAR-T patients' testimonials




