CAR-T success rate and activity overview in Sheba, Israel - 2024
The following information summarizes a lecture by Dr. Avigdor Abraham, MD, Director of the Institute of Hematology and Co-Director of the Division of Hematology and Bone Marrow Transplantation at Sheba Medical Center. This lecture was presented at the EBMT Conference in Seville, Spain, in February 2024. (see recording from minute 21:20)

Today, I would like to share with you our experience with point-of-care CAR-T cell therapy in a relatively large single-center cohort of patients. In many countries, the availability of CAR-T cell treatment is limited due to factors such as manufacturing constraints, regional access challenges, staffing shortages, and reimbursement issues. Another significant limitation of the approved product is the prolonged turnaround time, which poses a major problem for patients with rapidly progressing diseases.

As of now, three commercial anti-CD19 CAR-T cell products have received approval from the Israeli Ministry of Health. However, the commercial anti-BCMA CAR-T cell product has not been approved in Israel.
Additionally, three academic trials are being conducted in Israel, two of which involve anti-CD19 and anti-BCMA CARs, locally produced at Sheba Medical Center.
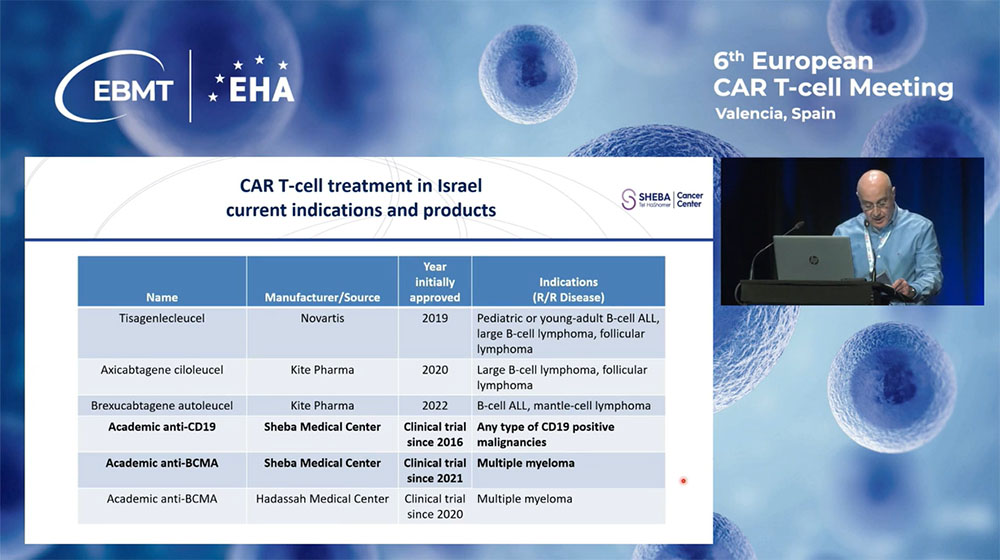
In 2016, three years before the release of the first commercial product in Israel, we began producing anti-CD19 CAR-T cells under phase 1b and phase 2 clinical trials for various hematologic malignancies.
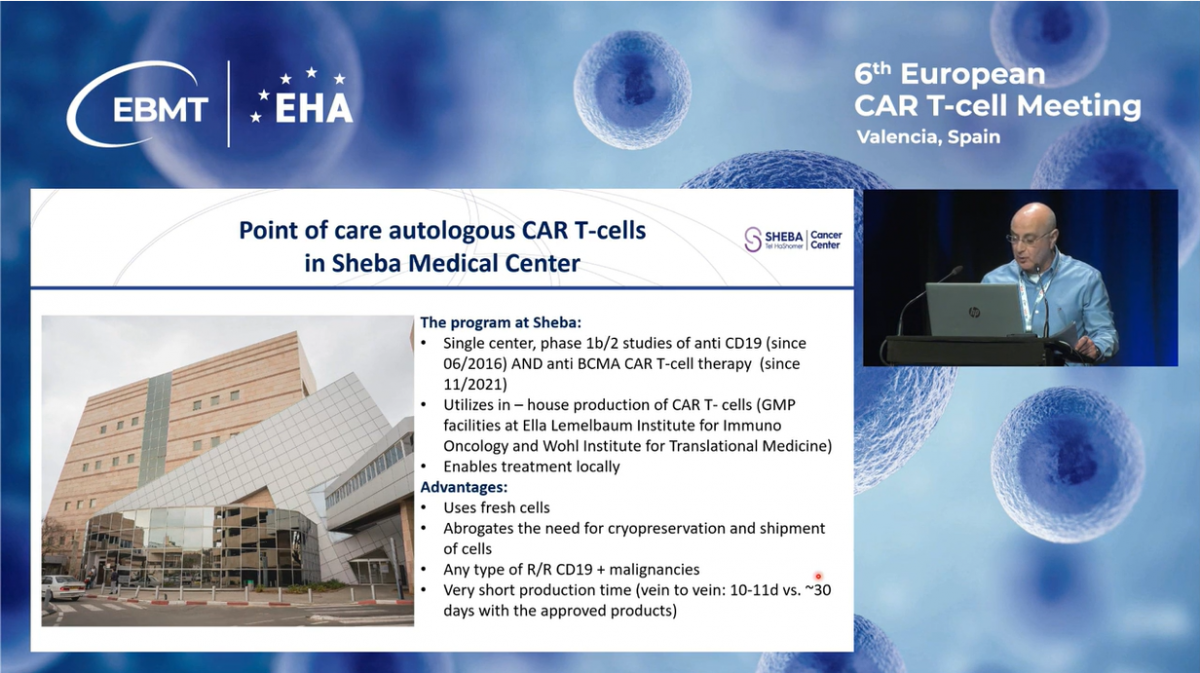
Since November 2021, we have also expanded our program to include anti-BCMA CAR-T. The CAR-T cells are produced manually in the GMP facilities at Sheba Medical Center. The advantages of local production include the use of fresh cells, which eliminates the need for CAR preservation and shipment, reduces treatment costs, and most importantly, shortens the vein-to-vein time to only 10 to 11 days. Our program allows treatment for almost any patient with CD19-positive malignancy, including some with no other therapeutic options.
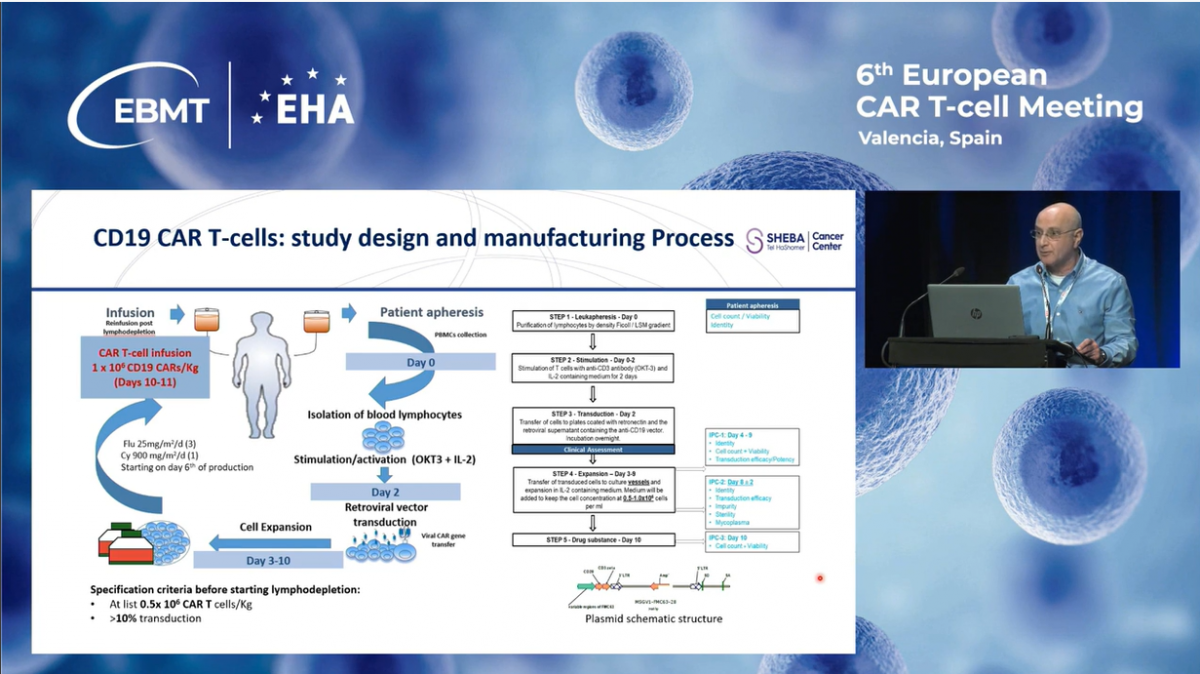
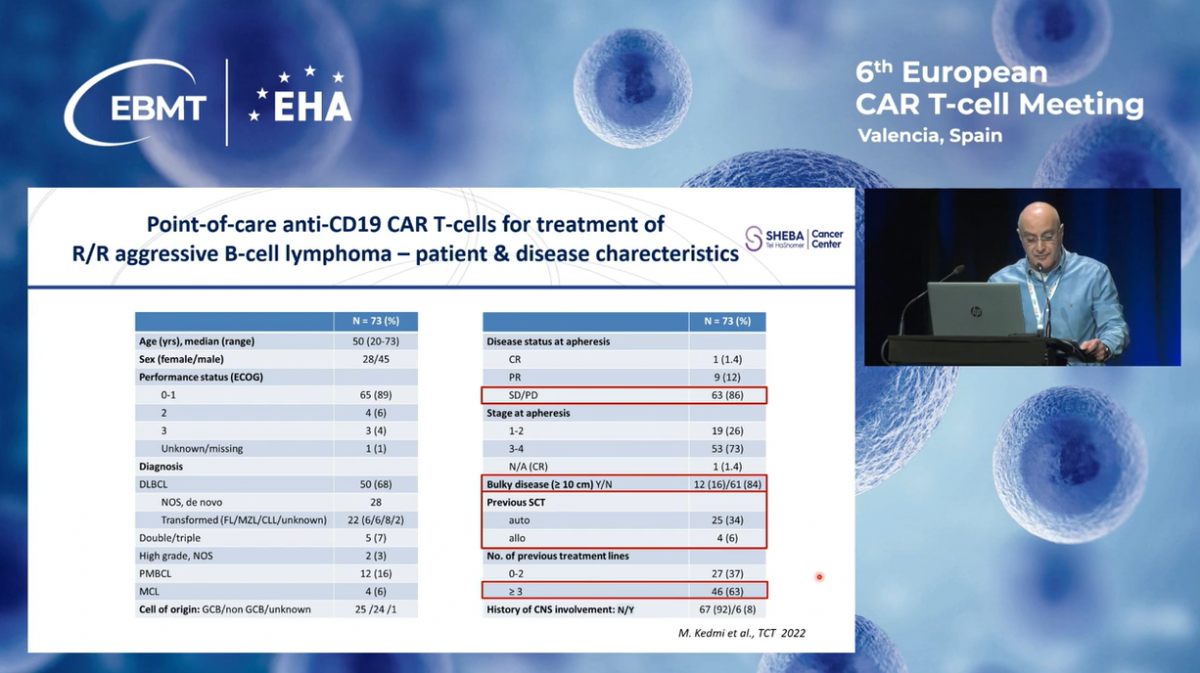
These are the key eligibility criteria of our program. Patients who had any malignancy expressing CD19. Eligible patients were either resistant to or relapsing after standard therapy, including those with relapse following transplantation.

The primary endpoints of this study were safety and overall response rates 28 days after the CAR infusion, and the secondary endpoints included overall survival and progression-free survival. In addition to this program, the availability of rapid production also allows us to provide salvage therapy to those patients without a specific commercial product.
We have published our experience with T-cell products following leukapheresis in a real-life setting.
This slide represents our experience with Sheba Point of Care CD19 CAR-T therapy.
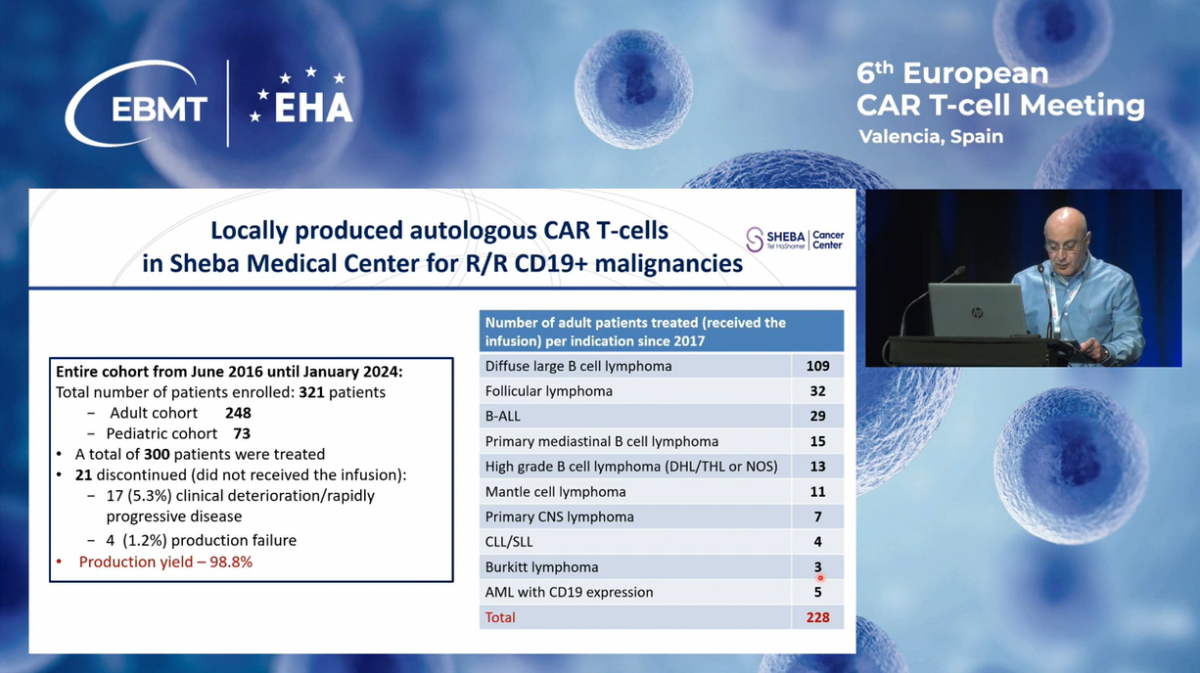
Until January 2024, a total of 321 patients with relapsed refractory CD19-positive diseases were enrolled, including 248 adults and 73 children. In the entire cohort, 21 patients did not receive the infusion; seventeen due to clinical deterioration or rapidly progressive disease, and only four because of production failures.
CAR-T for Multiple Myeloma
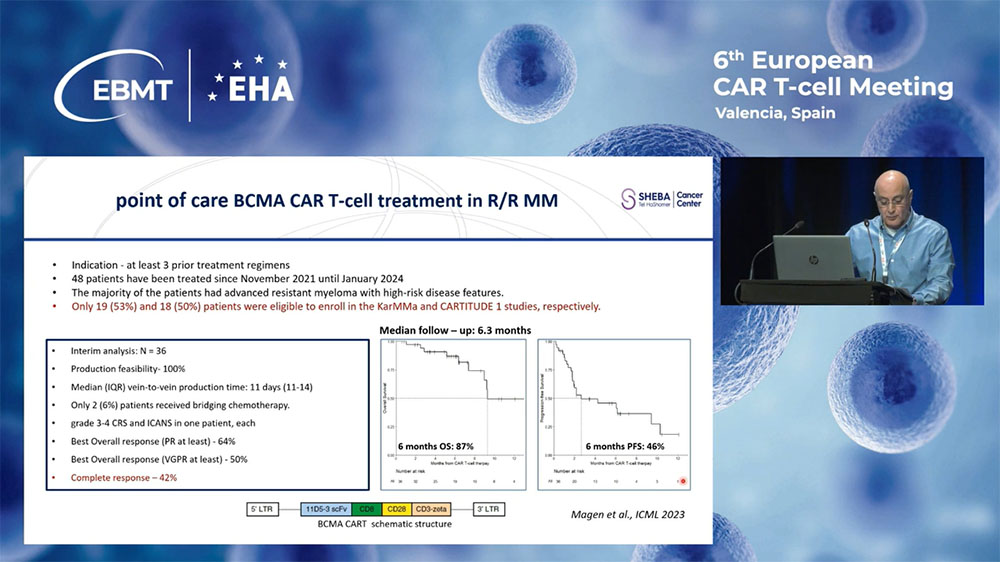
I would like to show you our preliminary data on the treatment of multiple myeloma with our locally produced BCMA CAR-T cells.
Since November 2021, we have also expanded our program to include anti-BCMA CAR-Ts, now given to relapsed refractory myeloma patients who have received at least three lines of treatments. The manufacturing process is identical to that for CD19 CARs. After 10 days of culture, patients receive nine million cells per kilogram of fresh CAR-T cells.
Until January 2024, we enrolled 48 patients. This is the interim analysis of the first 36 patients. The best overall response rate was 64%, with complete response achieved in 45%.
Summary of Sheba CAR-T Program Experience
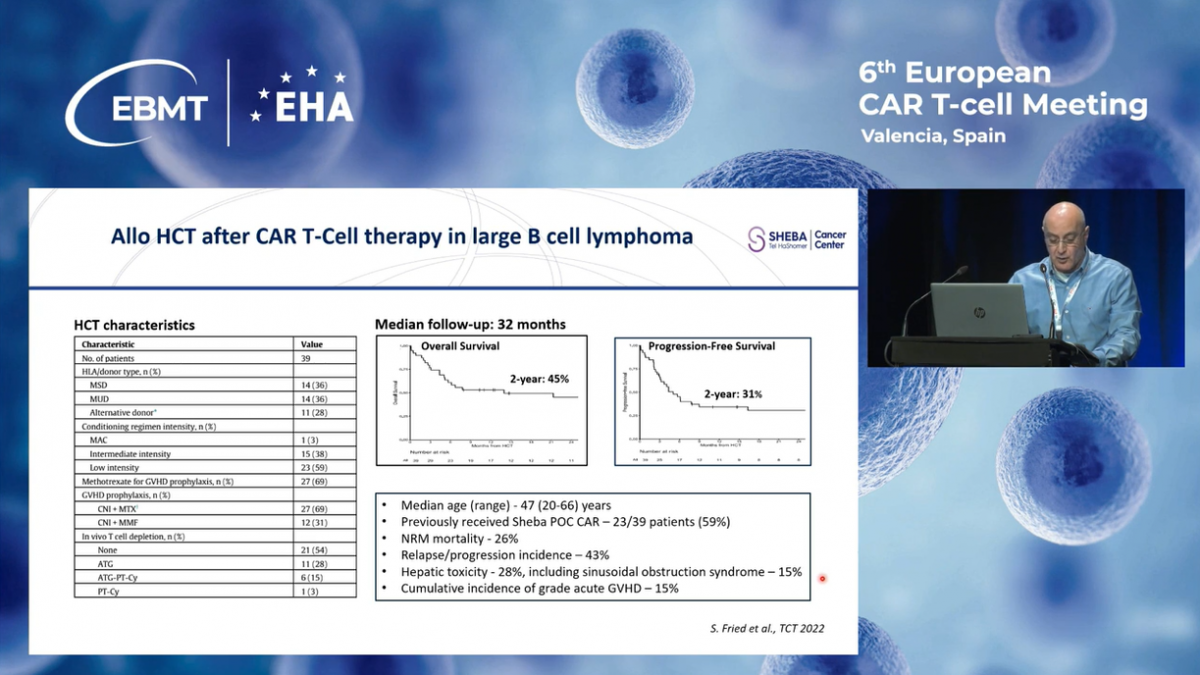
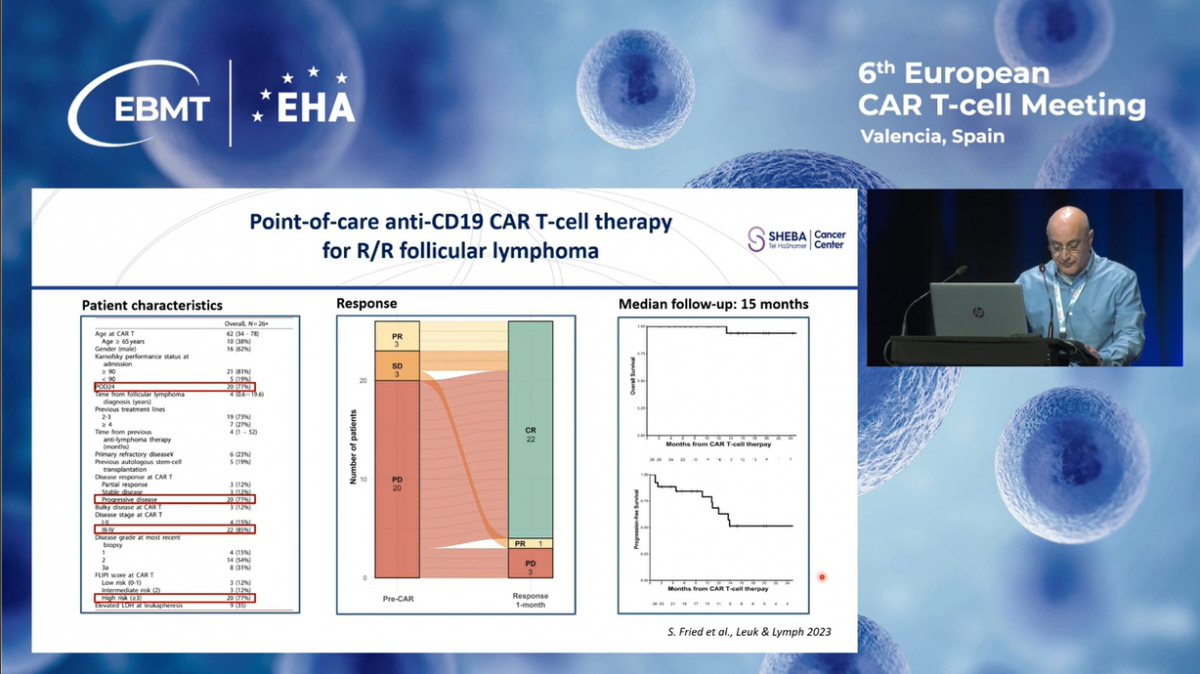
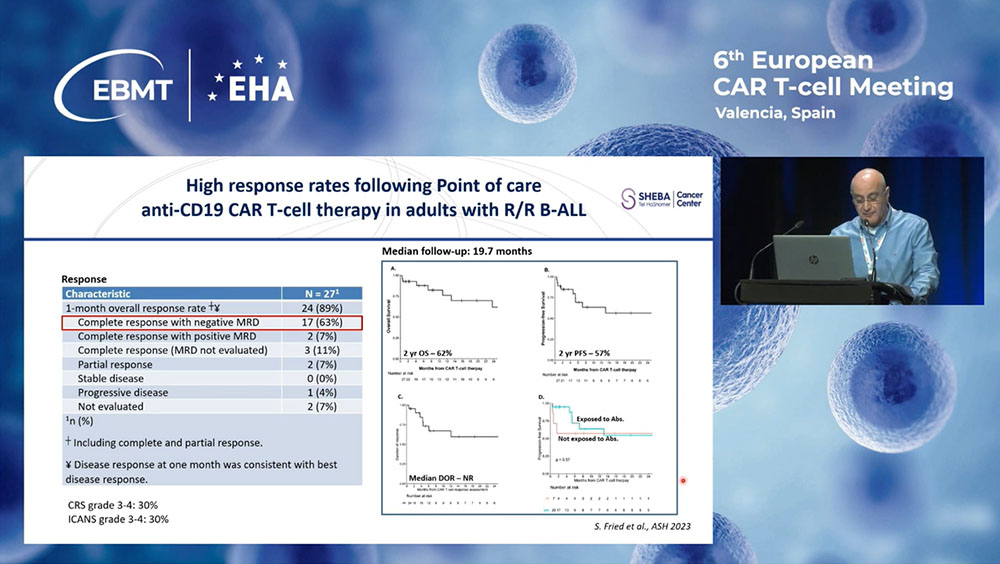
To summarize my presentation, treatment with our locally-produced CD19 CAR-T cells in patients with relapsed refractory, large B-cell lymphomas, follicular lymphoma, and B-ALL is well-tolerated, and the long-term outcomes are comparable to those reported with commercial products.
Our point-of-care BCMA practices induce good response rates with encouraging survival outcomes and an excellent safety profile in relapsed refractory multiple myeloma.
Point-of-care production is very rapid and efficient. It obviates the need for cell cryopreservation and product shipment. The short vein-to-vein time decreases the need for bridging therapy and associated treatment toxicity in most patients.
Finally, a new program with point-of-care production, using the fully-automated flow system of the cocoon, is also scheduled in the next few months.

CAR-T information:



