CAR-T cell therapy in Sheba: overview of activity– Prof. Arnon Nagler: part 2
This is a summary of Prof. Nagler’s lecture at the Sheba-React event in July 2023
Prof Arnon Nagler, M.D., M.Sc. is the of the President Hemato-Oncology Center in Sheba and Co chair Molecular markers subcommittee of the ALWP of the EBMT
Read part 1 >>
CAR-T for Myeloma: Sheba's results
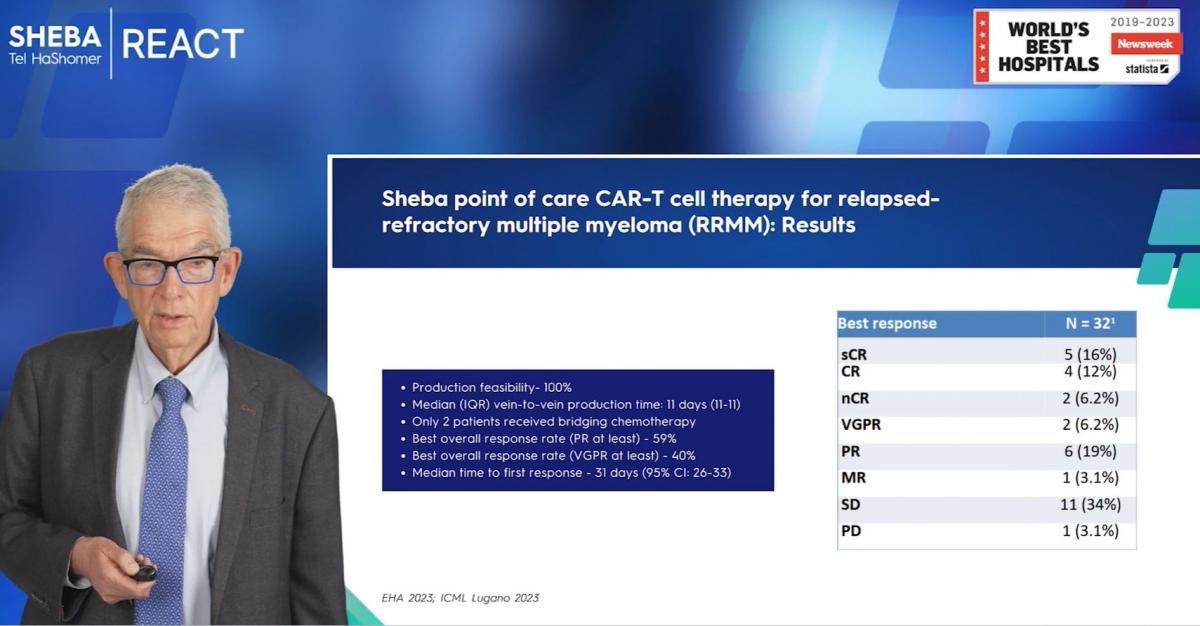
I had the pleasure of presenting our initial findings at the esteemed Lugano Conference just a few days ago. Thus far, we've treated 32 patients ranging in age up to 67 years. Our patient cohort comprises an advanced group, as evidenced by the fact that 53% of our patients were ineligible for the CMA study on commercial IDA cells, and another 16% were excluded from the UDE study on ilta cells. This includes those with double refractory, triple refractory conditions - certainly a challenging group.Nevertheless, our preliminary data reveals a remarkably low toxicity profile. We've recorded no grade three to four adverse events and, significantly, no deaths related to cellular therapy. Globally, the incidence of side effects such as CRs and icons in myeloma is low. Interestingly, our data shows even lower incidence in myeloma compared to lymphoma.
One noteworthy achievement is our success rate in CAR T-cell production. We've been able to successfully produce CAR T-cells in 100% of the treated patients. This is particularly impressive considering the complexity involved in treating myeloma patients post-transplantation and those receiving Melphalan therapy.
Our vein-to-vein time averages around 11 days. With respect to treatment outcomes, we achieved an overall response rate of 59%, and a 'very good' partial response rate of 40%. The median time to first response was recorded as 31 days.
To highlight a case, we had a 50-year-old female patient with unfavorable cytogenetic and prognostic indicators, and a history of plasmacytoma after three lines of therapy. Post CAR T-cell therapy, her PET CT scans came back clean, which is quite encouraging.
Although our follow-up time has been relatively short, these results are impressive, especially considering that most of our patients are ineligible for the two FDA-approved CAR T-cell therapies - IDA cells and CTA cells. We look forward to continuing our work and further assessing the potential of our treatment approach.
AML CAR-T
I'd like to share with you another piece of data from our studies - specifically regarding Acute Myeloid Leukemia (AML) with A 21 translocation. Admittedly, our research in this area is still in the early stages.As of today, we have treated six patients with this unique AML. The outcomes have been somewhat mixed: four of these patients responded to the treatment, but sadly, these responses were typically short-lived.
These findings were only recently presented. If we consider the 'swimmer plot' for these six patients, all with unique AML, A 21 translocation, and CD 19 expression, we see that four patients reached complete remission (CR). However, as noted earlier, this was transient.

Predicting Patient Response to CAR T-cell Therapy
The most pressing issue today, which is currently being addressed, involves predicting which patients will respond to CAR T-cell therapy. A study involving 177 patients from Sloan Kettering and 150 from Sheba divided patients into two groups: inflammatory and non-inflammatory. The patient demographics were similar across studies. Notably, the rate of complete response was lower. These basic observations may aid in predicting which patients will respond to CAR T-cell therapy, thereby improving outcomes for those who don't initially respond to this treatment.
Summary of Sheba’s academic CAR-T program
In this lecture, I've had the privilege of discussing with you our vibrant and highly productive academic CAR T-cell program at Sheba. We've treated several hundreds of patients, covering a wide range of lymphomas, some of which are not being addressed elsewhere.We're pioneers in treating both pediatric and adult Acute Lymphoblastic Leukemia (ALL) patients and even unique cases of Acute Myeloid Leukemia (AML). An integral part of our efforts has been dedicated to understanding which patients will respond to CAR T-cell therapy and which ones may not.
Perhaps the most crucial takeaway from our work is our strong belief in the potential of point-of-care CAR T-cells. This approach could significantly enhance accessibility and affordability of CAR T-cell treatments, offering a lifeline to patients who may have exhausted all other options.
CAR T-cell therapy has the potential to transform lives worldwide. Unfortunately, at present, it's mainly available in a few centers and primarily in affluent countries. We are working tirelessly to change this, to bring CAR T-cell therapy to patients everywhere who need it. Many such patients come to us, and we do our utmost to help them.
A look on the future of CAR-T
CAR T-cell therapy, I would argue, is the most significant recent development in the treatment of hematological malignancies related to the lymphatic system. Over the years, we have eagerly awaited this kind of immunotherapy. After all, an allogeneic transplant, while potentially the most powerful form of immunotherapy, comes with considerable toxicity and even mortality risk.CAR T-cell therapy, on the other hand, is a far more sophisticated form of immunotherapy and comes with significantly less toxicity. It can be administered to patients of all ages, even those with resistant or refractory diseases. While its efficacy might be somewhat reduced in patients with no disease or high-risk disease, it still proves to be effective in resistant diseases.
Notably, CAR T-cell therapy remains effective regardless of the presence of double or triple hits and high-risk genetics. Even though it may be slightly less potent, it's arguably more effective than transplants. This makes it a valuable treatment option for patients who have failed transplant procedures and for those who have no other available alternatives.
At present, CAR T-cell therapy is typically used as a last resort. However, as we begin to administer CAR T-cell therapy earlier in the treatment process, we expect to see improved outcomes. In lymphoma, for example, it's now approved for second-line therapy, and there are studies exploring its use as first-line therapy in high-risk lymphomas. In myeloma, the therapy has shown outstanding results, achieving high rates of complete remission and MRD negativity even in patients who have exhausted all other treatment options.
Looking ahead, we anticipate that CAR T-cell therapy will continue to revolutionize the treatment of lymphatic hematological malignancies. As for myeloid malignancies, the path is more challenging, and our progress is slower. While we've seen some success in very specific groups of patients and have studies ongoing with anti-CD 33 and others, we are still in the early stages of development in this area.
About the cost and affordability of CAR-T
In the medical field, we've witnessed numerous examples of sophisticated technologies becoming more affordable over time. For instance, deep sequencing, which initially cost millions of dollars per patient, now costs around a thousand dollars. Therefore, it's not unreasonable to expect similar cost reductions with CAR T-cell therapy, despite its complexity.For the first time, we've seen a sophisticated cellular therapy being mass-produced and delivered to clinics. It required pharmaceutical companies to establish new systems of collaboration with hospitals and transplant centers. We've faced challenges, such as the Covid pandemic, which slowed progress. However, as we gain more experience and develop more products, it's plausible to anticipate CAR T-cell therapy becoming more accessible and affordable.
While the cost and accessibility of these therapies aren't directly under my control, we are working to make these solutions more broadly available. As I mentioned, point-of-care CAR T-cells are being used and developed by an increasing number of centers. This does raise certain regulatory and authority-related challenges, but it's not impossible to overcome them.
I wouldn't label this approach as 'boutique medicine'. Rather, over time, I believe we'll see CAR T-cell therapies becoming more common, not just in large centers or wealthy Western countries. Hopefully, these revolutionary treatments will reach patients all over the globe. Read part 1 >>
CAR-T information:



